Previous McGeoch/Guidotti 1998 ONR/DARPA sensor research
POLYMERS 12.7 GIGA YEARS AGO
Julie E M McGeoch, High Energy Physics DIV, Smithsonian Astrophysical Observatory Center for Astrophysics | Harvard & Smithsonian 60 Garden St, MS 70 Cambridge MA 02138. USA. julie.mcgeoch@cfa.harvard.edu
Department of Molecular and Cellular Biology, Harvard University, 52 Oxford str NW140, Cambridge, MA 02138, USA. mcgeoch@fas.harvard.edu
Our research investigates ancient polymers composed of hydrogen, carbon, nitrogen and oxygen (polymer amides) with Fe, Si, O and Li:
1) Potentially forming the first material topology of the Universe:
Asteroid Polymer References:
McGeoch J. E. M. & McGeoch M. W. (2008) Entrapment of water by subunit c of ATP synthase. J. Roy. Soc. Interface 5, 311-318 doi:10.1098/rsif.2007.1146.
McGeoch J. E. M. and McGeoch M. W. (2014) Polymer Amide as an Early Topology. PLoS ONE 9(7): e103036. doi:10.1371/journal.pone.0103036
McGeoch J. E. M. and McGeoch M. W. (2015) Polymer amide in the Allende and Murchison meteorites. Meteoritics & Planetary Science 50, Nr12 1971-1983. doi: 10.1111/maps.12558.
McGeoch J. E. M. and McGeoch M. W. (2017) . A 4641Da polymer of amino acids in Acfer-086 and Allende meteorites. https://arxiv.org/pdf/1707.09080.pdf
McGeoch M. W., Šamoril T., Zapotok D. and McGeoch J. E. M. (2018) Polymer amide as a carrier of 15N in Allende and Acfer 086 meteorites. https://arxiv.org/abs/1811.06578 & 2023 Under review at International Journal of Astrobiology.
McGeoch M. W., Dikler S. and McGeoch J.E.M. (2020) Hemolithin: A Meteorite Protein containing Iron and Lithium. arXiv:2002.11688 [astro-ph.EP]
McGeoch M. W., Dikler S. and McGeoch J.E.M. (2021) Meteoritic Proteins with Glycine, Iron and Lithium [physics.chem-ph] & 2023 under review at Royal Society Open Science.
McGeoch J. E. M. and McGeoch M. W. (2021) Structural organization of space polymers. arXiv:2104.02731 [cond-mat.soft] Phys. Fluids 33, 067118
Julie E. M. McGeoch and Malcolm W. McGeoch. (2022) Chiral 480nm absorption in the hemoglycin space polymer.
Julie E. M. McGeoch and Malcolm W. McGeoch. (2022) Chiral 480nm absorption in the hemoglycin space polymer: a possible link to replication. Scientific Reports DOI : 10.1038/s41598-022-21043-4
Malcolm W McGeoch, Robin Owen, Sofia Jaho and Julie E M McGeoch (2023) Hemoglycin visible fluorescence induced by X-rays. J. Chem. Phys. (in press) (2023); https://doi.org/10.1063/5.0143945
Julie E M McGeoch., Anton J Frommelt, Robin Owen, Gianfelice Cinque, Arthur McClelland, David Lageson and Malcolm W McGeoch. (2024) Fossil and present-day stromatolite ooids contain a meteoritic polymer of glycine and iron. Submitted & arXiv:2309.17195 [physics.geo-ph]
Julie E. M. McGeoch and Malcolm W. McGeoch (2024) Polymer amide as a source of the cosmic 6.2 micron emission and absorption arXiv:2309.14914 [astro-ph.GA]. MNRAS DOI: 10.1093/mnras/stae756.
Harvard-Heidelberg Workshop October 16-20th 2023. IR absorption of hemoglycin via quantum/experimental/future JWST at 6.2µm.
Cfa SAO ITC Lunchtime talk December 7th 2023. A space polymer of glycine and iron – its potential role in accretion and detection via JWST MIRI at 6.21µm: https://youtube.com/live/fkcuflI7_FI
UPDATE of research March 25th 2024
The space polymer hemoglycin of Glycine18 Hydroxy-glycine4 Fe2O4 has been analyzed by mass spectrometry, X-ray diffraction (APS Argonne National Laboratory beam line 31-1D-D and Diamond Light Source beam line I24) and isotope analysis (mass spectrometry and FIB/SIMS). The source material for the polymer is the meteorites Acfer-086, Allende, Efremovka, Kaba, Orgueil and Sutter’s Mill. Murchison was analyzed but did not contain the polymer.
In clean rooms the fresh faces of the meteorite samples are etched to produce micron scale particles (Acfer-086, Allende, Efremovka and Kaba) or larger 100mg scale particles (Sutter’s Mill and Orgueil).
In samples that are highly carbonaceous with a loose topology like Orgueil and Sutter’s Mill it is possible with a micro-scalpel blade to free individual 500µm scale crystals from dry particles, their destination being X-ray diffraction analysis at the synchrotrons APS and Diamond. All other meteorite samples including Orgueil and Sutter’s Mill are subjected to Folch extraction. Basically a 2 phase Folch system (chloroform:methanol:water ~ 3.2:2:1) allows solvation of hemoglycin which diffuses from the meteorite sample into the interphase layer of the Folch phase system. The bottom dense phase is chloroform and the upper polar phase methanol and water. Hemoglycin is less dense than chloroform but denser than methanol/water and therefore hemoglycin collects at the interphase.
Hemoglycin, whatever the meteorite source, collects very quickly, within minutes to hours at the interphase of the Folch extraction. It takes months for most hemoglycin crystals to form as the Folch system in the V-vial undergoes slow evaporation, but mass spectrometry can be performed within hours of solvation in sample aliquots taken from the interphase layer.
The structure of hemoglycin in its source protoplanetary disk was probably mostly a low-density extensive lattice but after Folch solvation and slow crystallization other topologies emerge:
- The low-density lattice with the equivalent crystal being a dark hexagon – this probably formed in the meteorite.
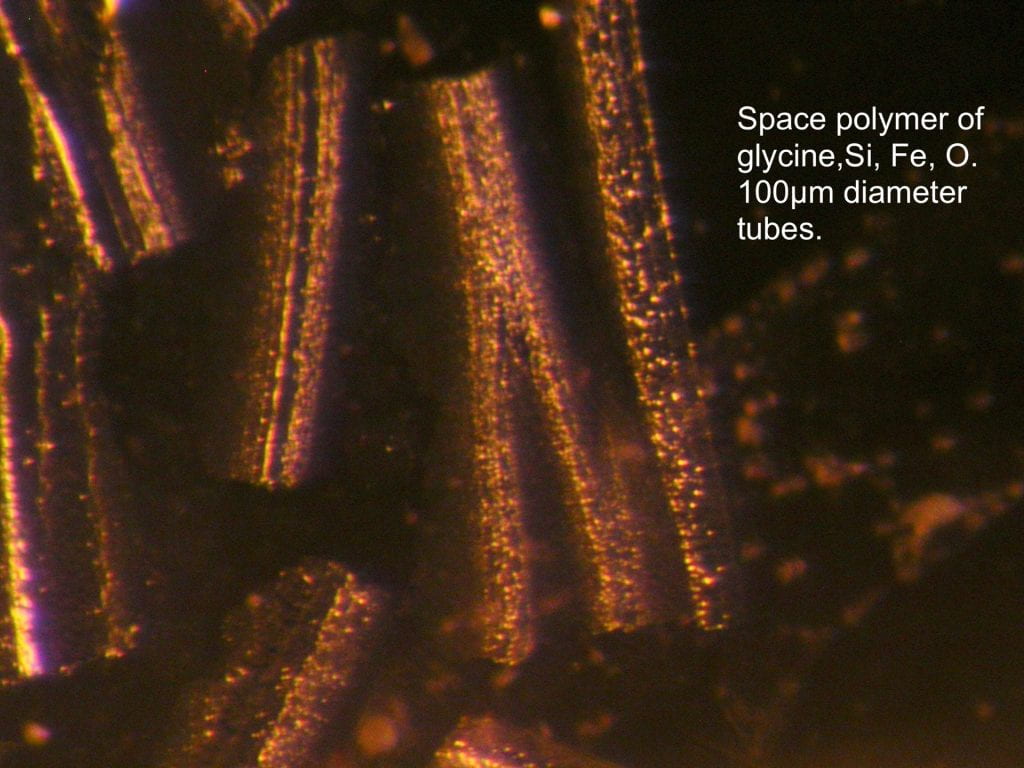
- A 2-D flat sheet that rolls up into tubes with the equivalent crystal being an amber fiber.
- Bright reflecting, colorless, spheres surfaced by hexagons and pentagons that entrap either water or a gas with the equivalent crystal being 50µm or smaller spheres, often within the 2D tubes. These hemoglycin spheres are on the micron scale not the nm scale of carbon “bucky-balls”. The meeting points of the hexagons and pentagons are trios of Fe atoms with oxygens hence the rods connecting vertices are the length of one hemoglycin unit, i.e. 5nm. This accounts for the large size of the spheres which are stable and can to some degree be compressed to a flattened lenticular shape when incorporated into a flat crystal.
The entrapping spheres of hemoglycin, the 3rd listed topology, may be an important topology in a protoplanetary disk because these disks go through cycles of heating and cooling and changes in water content. An entity like hemoglycin that could retain the water would be valuable to a chemistry system needing this solvent. The variety of hemoglycin topologies leads to it having great flexibility in adaptation to different degrees of hydration.
In Hartree-Fock calculations the polymer has an absorption of blue light at 480nm that is dependent on rectus “R” (= dextro D) chirality in a hydroxy-glycine residue whose C-terminus is bonded to an iron atom. Experimentally this has now been measured at the Diamond Light Source UK. The absorption originates in the Fe II state as a consequence of chiral symmetry breaking. Hemoglycin also absorbs IR light which is important when the polymer is in a molecular cloud, that is not yet a proto-planetary disk with a sun providing visible wavelengths. The polymer core unit could have been selected 4.5 billion years ago in our protoplanetary disc by blue light from the early sun.
Hemoglycin could be responsible for protein on Earth or other planets having S/L chiral amino acids. Hemoglycin can form in R or S chirality within the C-terminus amino acid adjacent to Fe. The 480nm absorption can power a reaction in which water is converted to hydrogen peroxide and the hydroxyglycine is converted back to glycine. The R form is thereby continually destroyed whereas the S form remains available for the production of amino acids through condensation reactions.
Besides being present in carbonaceous meteorites hemoglycin has also been extracted and crystalized from 2 fossil stromatolites and is possibly in a present day stromatolite from Shark Bay Australia. the fossil stromatolites formed on Earth 2.1billion years ago. Potentially the fossil hemoglycin was delivered during the Late Heavy Bombardment (LHB) to Earth. Data to support this being the hemoglycin in a fossil has extra-terrestrial isotopes similar to that in meteorites. The polymer on the Precambrian Earth could have functioned to drive the Great Oxygenation Event beginning 2.4Gya by splitting water in response to ultraviolet irradiation. Also, it could provide an energy source to early biochemistry and/or it simply delivered a source of polymer glycine.
Hemoglycin in stromatolites is interesting because its role in the stromatolite topology is abiotic. Its action is likely to involve light and both visible and IR light absorption have been measured in ooids of stromatolites. Hemoglycin could be a most basic molecular entity supplying energy to the early Earth before a biochemical entity arose 2-3billion years ago. Hemoglcyin arrived on the early Earth via in-fall and at that early time asteroid material was delivered at the rate of 1e12 kg/yr. Now in present times the asteroid derived material delivery rate is less at 1e5 kg.yr.
We are submitted a proposal to detect hemoglycin in cycle 4 on the James Web Space Telescope (JWST). The JWST will point at 2 stars in our galaxy using them to backlight molecular clouds that potentially contain hemoglycin and will absorb at 6.2µm.
BACKGROUND FACTS:
The Universe started 13.73±0.12Gyrs ago (chemical time line graph) (Komatsu et al, 2008, Five-year Wilkinson Microwave Anisotropy Probe (WMAP) observations: Cosmological interpretation. Astrophysical J 2008arXiv0803.0547K).
At 500million years from the start of the Universe the constituent elements of polymer amide had formed but not the halogens which in molecular configurations, had they formed at that very early time point, could have readily solubilized polymer amide molecules.
Therefore it follows that in episodes of relatively lower temperature and higher density in the Universe, polymer amide could have formed from Hydrogen, Carbon, Nitrogen and Oxygen, Fe, Si, Li just 500million years after the start of the Universe.
The order of events from the start of the Universe up to the point when polymer amide could in principal have started to form are as follows:
FIRST 500million Years
- Start (BB) at 13.73±0.12Gyrs ago.
- First nucleosynthesis forms hydrogen and helium which are accreted via gravity to form large First Generation (Pop 111) Stars
- Another nucleosynthesis in these stars formed the first 8 elements: H, He, Li, Be, B, C, N, O (Fe, Si, Li, Be & B were very low in amount relative to H, C, N, O). These elements were dispersed outwards when the first stars exploded.
- Molecules could form from chance encounters of these elements at the edge of Pop 111 star dispersal boundaries.
- Potentially the formation of a material topology (via the molecules that the elements could form) would have been a key event in the early molecule survival. Once any material topology arose it would have provided a micro-environment that favoured via confinement further element interactions to form molecules.
- Most importantly water and short stretches of polymer amide might have co-formed* i.e. without polymer amide no bulk water formed and without water no polymer amide formed. This bulk water was explicit water from the condensation reaction during the amide bond formation. Gas phase separate water molecules in space could form and not be dependent on co-formation from polymer amide but the start of “bulk water” might have needed to be tethered by intermolecular hydrogen bonds (water to water and water to amide) to start its bulking process. The polymer acted as a container or surface to allow the tethering. Once some bulk water has formed other separate gas phase water could join or nucleate to the intial explicit water.
The key Universe event is therefore that which allowed the first material topology to arise. In relation to theoretical astrophysics it could also be considered to potentially have provided a material based interface for “String Theory”.
This subject depends on the juxta-positioning of astrophysical theory relating to events 13Gyrs ago and very recent Earth based experimental evidence. Because we had the fortune to observe in 2007 during a very basic experiment involving a pure preparation of an ancient amide, its exceptional properties that immediately suggested to us its early role in the Universe, we are introducing here the predicts of our experimental observations. However this does not mean that these observations in any way prove that proteolipid which is a hydrophobic polymer amide was the first material topology of the Universe. The observations support this hypothesis, because the entrapped water oxygens (by a proteolipid skin) have <1pm of error in their alignment which indicates from chemical, engineering and physics standpoints, a highly significant statistical selection of a system. Further the spacing distance between those oxygens is 3.7Å and slightly at right angles to this, the carbon backbone of the proteolipid skin has also the same 3.7Å spacing. If there was a co-formation of water and polymer amide 13Gyrs ago*, this identical spacing distance within the structure of 2 distinct abuting molecules could indicate the precise structural start point of the co-formation process.
BACKGROUND OBSERVATIONS AND EXPERIMENTAL EVIDENCE:
Water entrapped by a self-replicating membrane of polymer hydrophobic protein has been suggested as the first material based topology of the Universe (McGeoch 2008 Harvard da Vinci Group, May). At those early times it was the formation of the first material before any other existed that was important. Ice nucleation (IN) is discussed as an early accretion process in the Universe, which could either follow after the establishment of the first topology or the data on IN in reality includes ice in a mixed topological configuration i.e. it is not just water but water plus polymer amide.
That a similar system existed when cells first arose on Earth 3.8 Gyrs ago, is supported by evidence that the nucleotide code of one hydrophobic polymer, proteolipid, is the most conserved throughout Archaea, Eubacteria and Eukaria. Water-tight hydrogen bonded beta-sheets of proteolipid can stack as 6Å deep layers around centrally entrapped, ordered water, providing a topology that separates charge, and intermittent alpha-helical configurations confer ion channel function for the transport of cations and water between the layers (McGeoch & McGeoch 2008 J. Roy Soc Interface 5,311-318).). Here we hypothesize that this ancient system of separating charge between insulating layers of hydrophobic protein was incorporated into the first cells as the fundamental component for information storage, later becoming a nervous system. The advent of nucleotide-code-based cell chemistry evolved proteins to interact with this system, some of which suppressed its inclination to self-replicate from a template. Today certain diseases of the nervous system in Homo sapiens involving aggregated beta-sheet protein might have their basis in age-related imperfect transcription/translation or code mutations, rendering the code-based system of control, aberrant. We suggest that Battens (NCL’s), and Alzheimer’s disease might be in this category. The ratio of cell proteolipid mass to its gene codons P1, P2 & P3 translationed product should be higher than that for another control polymer like a skin keratin, if there is ancient template-based self replication for proteolipid, and in Batten’s Disease it should be even higher due to aberrant control of the template system.